The Avogadro's number is a constant used in analytical chemistry to quantify the number of particles or microscopic entities from macroscopic measurements such as mass. It is very important to know this number in order to understand molecule composition, interactions and combinations. Avogadro's number is named after an Italian Scientist, Amedeo Avogadro in the 19 th Century. As per his theory, at certain pressure and temperature conditions, the volume of gas is proportional to the number of molecules in the gas irrespective of the nature of gas.
- The Avogadro constant is named after the Italian scientist Amedeo Avogadro (1776–1856), who, in 1811, first proposed that the volume of a gas (at a given pressure and temperature) is proportional to the number of atoms or molecules regardless of the nature of the gas.
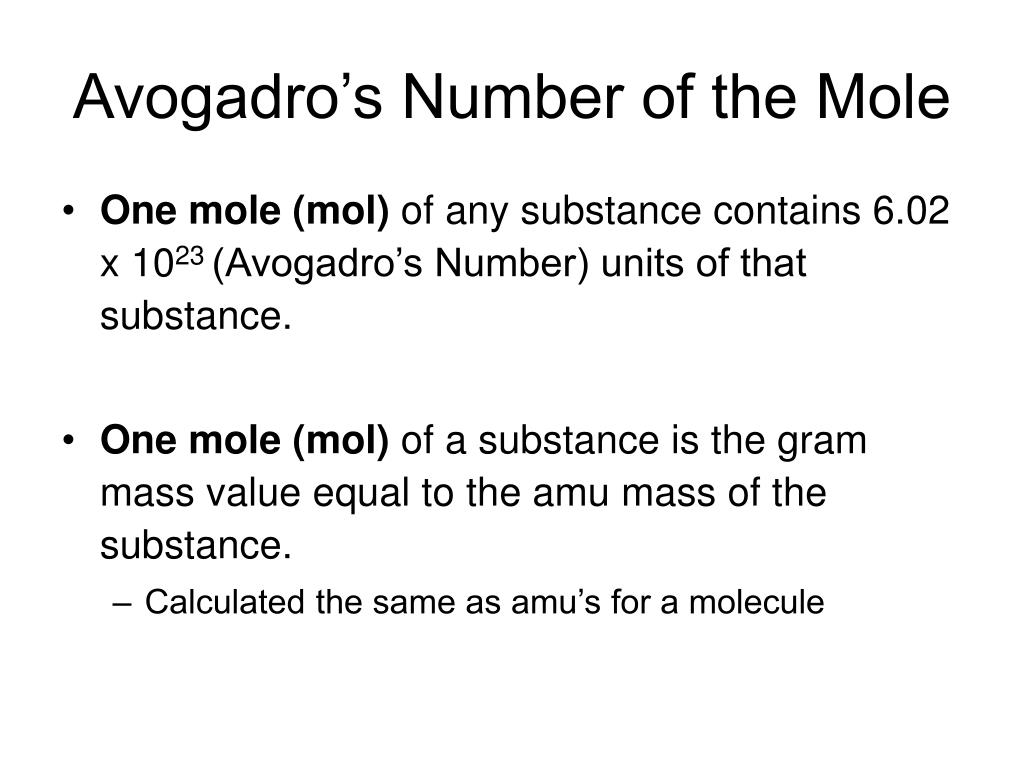
- Definition Avogadro's number is formally defined as the number of carbon-12atoms in 0.012 kg of carbon-12. It is approximately 6.022 × 1023particles/mole. Historically, carbon-12 was chosen as the reference substance because its atomic mass could be measured particularly accurately.
- Avogadro's Number is the number of Atoms or Molecules in one mole of a substance. It's value is estimated to be 6.022 x 10^23 for ease of calculation. However, the exact value of Avogadro's Number is 9072 The accurate determination of Avogadro's Number was possible due to Robert Millikan, an American Physicist.
Article media libraries that feature this video:Avogadro's number, Avogadro's law, Gas laws, Mole, Perfect gas law
Avogadro Number Meaning In Urdu

Meaning Of Avogadro's Number
Transcript
Avogadro's Number Song
Although we can’t see them, we are surrounded by gas molecules.
If we look at gas molecules in a balloon, we would see the gas molecules in motion, constantly bouncing up against the inside surface of the balloon.
How can we use math to understand gas in a closed system like this? The Ideal Gas Law states that for any gas, its volume (V) multiplied by its pressure (P) is equal to the number of moles of gas (n) multiplied by its temperature (T) multiplied by the ideal gas constant, R.
PV=nRT
But what exactly is R? If we rewrite the ideal gas law to solve for R, we find that R equals the value of P times V, divided by the value of n times T.
Because R is a constant, we can use the qualities of any gas — its temperature, pressure, volume, and number of moles — to determine the value of R. Avogadro’s law states that equal volumes of all gases, at the same temperature and pressure, have the same number of molecules.
This means four balloons, at the same temperature, filled to the same volume, with four different gasses, all contain the same number of moles of gas.
People have used this law to find the number of molecules of gas at a standard temperature and pressure, abbreviated as STP. STP is 273 Kelvin and 1 atmosphere (atm), the standard unit for atmospheric pressure. At STP, 1 mole of gas takes up 22.4 liters.
Let’s plug those numbers into the ideal gas law. R equals the value of 1 atmosphere multiplied by 22.4 liters, divided by the value of 1 mole multiplied by 273 degrees kelvin equals 0.0821 atmosphere liters per moles Kelvin.
Whew! Now that we know the value of R, we can use it the next time we need to estimate the behavior of gas in an ideal situation.
If we look at gas molecules in a balloon, we would see the gas molecules in motion, constantly bouncing up against the inside surface of the balloon.
How can we use math to understand gas in a closed system like this? The Ideal Gas Law states that for any gas, its volume (V) multiplied by its pressure (P) is equal to the number of moles of gas (n) multiplied by its temperature (T) multiplied by the ideal gas constant, R.
PV=nRT
But what exactly is R? If we rewrite the ideal gas law to solve for R, we find that R equals the value of P times V, divided by the value of n times T.
Because R is a constant, we can use the qualities of any gas — its temperature, pressure, volume, and number of moles — to determine the value of R. Avogadro’s law states that equal volumes of all gases, at the same temperature and pressure, have the same number of molecules.
This means four balloons, at the same temperature, filled to the same volume, with four different gasses, all contain the same number of moles of gas.
People have used this law to find the number of molecules of gas at a standard temperature and pressure, abbreviated as STP. STP is 273 Kelvin and 1 atmosphere (atm), the standard unit for atmospheric pressure. At STP, 1 mole of gas takes up 22.4 liters.
Let’s plug those numbers into the ideal gas law. R equals the value of 1 atmosphere multiplied by 22.4 liters, divided by the value of 1 mole multiplied by 273 degrees kelvin equals 0.0821 atmosphere liters per moles Kelvin.
Whew! Now that we know the value of R, we can use it the next time we need to estimate the behavior of gas in an ideal situation.
