
Simple distillation is a method for separating the solvent from a solution, leaving behind the solute. This method works because the solvent has a much lower boiling point than the dissolved solute. Heating the solution allows the solvent to evaporate, and then it passes through a condenser, where it is cooled and condensed into a separate container. The solute does not evaporate and so it stays behind.
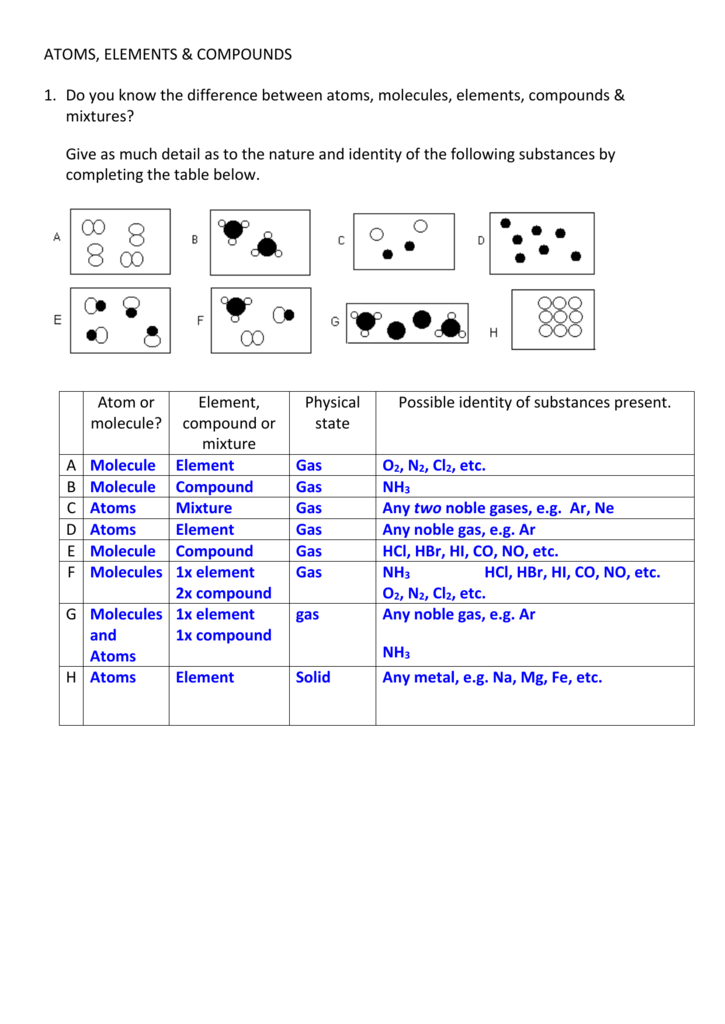
Elements and compounds. Those molecules which are made from only one type of atom are called ELEMENTS. Those molecules which are made up of different types of atoms are called COMPOUNDS, such as the molecule of water. Oxygen (O2), element made up of 2 atoms of oxygen. ATOMS, ELEMENTS, AND COMPOUNDS Unit Overview Introduction Atomic theory and its associated findings form the basis for modern chemistry. Building on past explorations using various substances and the particle model of matter, students should become familiar with the basic constituents of atoms and molecules, with chemical.
Fractional distillation is a method of separating multiple liquids from each other. The technique works in the same way as distillation, but on a much larger scale.
Filtration is a method for separating an insoluble solid from a liquid. A mixture of liquid and solid is passed through filter paper into a flask below. The solid stays on the filter paper (residue), and the liquid passes through to the container below (filtrate).
Crystallisation/evaporation is a method that separates a soluble solid from a liquid. A solution of liquid and dissolved solid is heated, causing the solvent to evaporate and leaves solid crystals behind.

Atoms Elements And Compounds Pdf
What is the simplest way of explaining what atoms, elements, compounds and mixtures are?
Atoms are the smallest bits of ordinary matter and are made from particles called protons (which carry a positive electrical charge), neutrons (which carry no electrical charge) and electrons (which carry a negative electrical charge). The protons and neutrons cluster together in the central part of the atom, called the nucleus, and the electrons 'orbit' the nucleus. A particular atom will have the same number of protons and electrons and most atoms have at least as many neutrons as protons.
An element is a substance that is made entirely from one type of atom. For example, the element hydrogen is made from atoms containing just one proton and one electron. If you had very, very good eyes and could look at the atoms in a sample of hydrogen, you would notice that most of the atoms have no neutrons, some of them have one neutron and a few of them have two neutrons. These different versions of hydrogen are called isotopes. All isotopes of a particular element have the same number of protons, but can have different numbers of neutrons. If you change the number of protons an atom has, you change the type of element it is. If you change the number of neutrons an atom has, you make an isotope of that element. All known elements are arranged on a chart called the Periodic Table of Elements.
A compound is a substance made from two or more different elements that have been chemically joined. Some examples of compounds are water (H2O), table salt (NaCl), table sugar (C12H22O11) and chalk (CaCO3).
A mixture is a substance made by combining two or more different materials in such a way that no chemical reaction occurs. A mixture can usually be separated back into its original components. Some examples of mixtures are a tossed salad, salt water and a mixed bag of M&M's candy.
Author:
Atoms Elements Compounds And Mixtures
Steve Gagnon, Science Education Specialist (Other answers by Steve Gagnon)
Related Pages:
For questions about this page, please contact Steve Gagnon.
